Since the 1950’s the theory of muscle force production, the Sliding Filament Theory, has been the accepted model for how muscles work. If you never studied it in school, I suggest you watch this video. It certainly isn’t part of most yoga teacher training programs.
Even if you didn’t study the mechanism behind how a muscle produces force in an academic setting, I’m guessing the overall message has helped form your understanding of muscle. I am confident in this assumption because I lead yoga teacher training programs and am constantly fielding questions that reflect the shortcomings of the Sliding Filament Theory – even if they are not asked in scientific language.
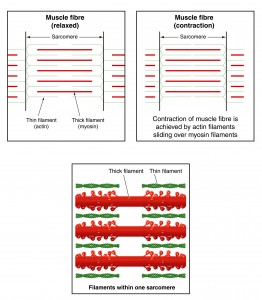
The Sliding Filament Theory assumes that force production is dependent on cross-bridge formation. This model works well when the muscle is contracting at resting length (isometric contraction) and particularly when the muscle shortens (concentric contraction).
Click image to enlargen (Image via Dreamstime)
But we’ve all heard of eccentric contractions, where the muscle lengthens while still producing force. The number of possible cross-bridge formations is diminishing, yet the muscle is still capable of producing force. Muscles can even produce force in the absence of cross-bridge formations, when muscle is stretched beyond the thick and thin filament overlap [1].
While the literature is very candid about our failure to understand eccentric contractions, textbooks and science teachers seem to throw muscle physiology at us, thereby overwhelming us with the details of a concentric contraction and diverting our attention away from the unexplainable eccentric contraction.
Why does this matter?
Because the focus on the mechanism behind concentric and isometric contractions lead to a public perception that muscles shorten when producing force. This gives us a very narrow view of how muscles actually work. Yoga teachers may associate shortening muscles as “on” and lengthening muscles as “off” – which translates into the idea that stretched muscles are “letting go.”
But this is far from the truth. Lengthening muscles may indeed produce force, and may even increase force production after an eccentric contraction [2, 3, 4, 5]. Stretching, it seems, does not require relaxation.
Several proposed theories attempt to explain this behavior, all of them subject to a fair share of both support and criticism. None of the theories are able to fully satisfy the explanation nor can they be fully dismissed [3].
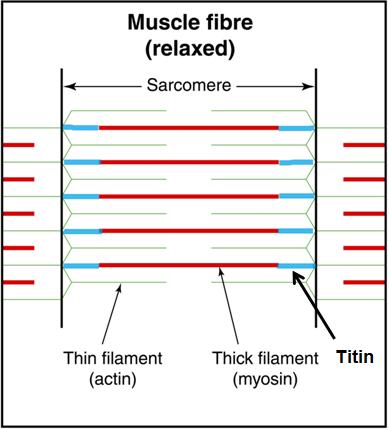
Titin, however, seems to be an essential missing link in how muscles actually work. Titin is a third filament, in addition to the thick filament (myosin) and the thin filament (actin). [1, 2, 3, 4, 6, 7]. Titin is an elastic protein surrounding the thick (myosin) filaments and continuing to the Z lines, anchoring myosin to the ends of the sarcomeres.
Click image to enlargen
Originally, titin was thought to passively control myosin position during contraction by providing passive tension during elongation [8,9], receiving the tensile load and holding the sarcomeres together during a “passive” stretch when the sarcomeres are not producing tension via cross-bridging. Titin, also subject to viscoelastic phenomena, creeps, and has limited mechanical range.
More recently, however, titin is thought to play an active role in muscle force production when the sarcomere is stretched beyond the active myosin-actin overlap (cross-bridge). Titin has not only been shown to increases stiffness by binding with calcium (Ca2+), but is also concluded to bind and interact with actin, forming a force producing interaction in the absence of cross-bridges [1, 3, 7, 10, 11].
Some researchers are even proposing a Three Filament Model (actin, myosin, and titin) as a more complete theory of muscle contraction [8]. This research challenges the popular belief that a relaxed muscle is better equipped to stretch and elongate, and suggests that contractions during a stretched state are much more complicated than the current paradigm depicts.
These discoveries certainly challenge the agonist-antagonist relationship we learned in the anatomy segment of our yoga teacher training, further challenging the 2-dimensional model of movement. Dare I say “3-D” without flexing my “guru” muscle?
It may take several decades for the new theory to make its way to movement professionals. But when it does, I expect the public perception of muscle function (and stretching) will be transformed and elevated.
________________________________________________________________________________
[1] Leonard, T. R., & Herzog, W. (2010). Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. American Journal of Physiology. Cell Physiology, 299(1), C14–C20. doi:10.1152/ajpcell.00049.2010
[2] Edman, K. (2012). Residual force enhancement after stretch in striated muscle. A consequence of increased myofilament overlap? The Journal of Physiology, 590(6), 1339–1345. doi:10.1113/jphysiol.2011.222729
[3] Minozzo, F., & Lira, C. (2013). Muscle residual force enhancement: a brief review. Clinics, 68(2), 269–274. doi:10.6061/clinics/2013(02)R01
[4] Rassier, D. E. (2012). The mechanisms of the residual force enhancement after stretch of skeletal muscle: non-uniformity in half-sarcomeres and stiffness of titin. Proceedings of the Royal Society, 279(1739), 2705–2713. doi:10.1098/rspb.2012.0467
[5] Shim, J., & Garner, B. (2012). Residual force enhancement during voluntary contractions of knee extensors and flexors at short and long muscle lengths. Journal of Biomechanics, 45(6), 913–918. doi:10.1016/j.jbiomech.2012.01.026
[6] Leonard, T. R., DuVall, M., & Herzog, W. (2010). Force enhancement following stretch in a single sarcomere. American Journal of Physiology. Cell Physiology, 299(6), C1398–C1401. doi:10.1152/ajpcell.00222.2010
[7] Powers, K., Schappacher-Tilp, G., Jinha, A., Leonard, T. R., Nishikawa, K., & Herzog, W. (2014). Titin force is enhanced in actively stretched skeletal muscle. The Journal of Experimental Biology. doi:10.1242/jeb.105361
[8] Alter, M. J. (2004). Science of Flexibility (3rd ed.). Human Kinetics.
[9] Herbert, R. (2005). How muscles respond to stretch. In K. Refshauge, L. Ada, & E. Ellis (Eds.), Science-Based Rehabilitation (pp. 107–130). Elsevier Limited.
[10] Herzog, W., Leonard, T. R., Joumaa, V., DuVall, M., & Panchangam, A. (2012). The three filament model of skeletal muscle stability and force production. Molecular & Cellular Biology, 9(3), 175–191.
[11] Nishikawa, K. C., Monroy, J. a, Uyeno, T. E., Yeo, S. H., Pai, D. K., & Lindstedt, S. L. (2012). Is titin a “winding filament”? A new twist on muscle contraction. Proceedings of the Royal Society. Biological Sciences, 279(1730), 981–990. doi:10.1098/rspb.2011.1304
Extend Your Learning: Online Education With Jules
Yoga Biomechanics Livestream
My flagship 3-day livestream course is for teachers who have an insatiable curiosity about human movement and kinesiology, are eager to know what the research says about yoga, and are open to accepting that alignment rules aren’t always accurate. Includes 30 days of access to the livestream replay and slides. 18 CEUs. Learn more >


Thank you for all of your posts. I appreciate all the hard work you have done, and continue to do, but what I love most is that you SHARE it with us!!!
I am grateful for you!!!
Anita Smith